For plant and animal immune systems the similarities go beyond sensing
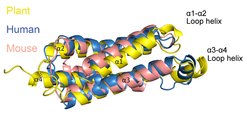
Superposition of the HeLo domains of plant (yellow), human (blue), and mouse MLKL (pink).
Although profoundly different in terms of physiology, habitat and nutritional needs, plants and animals are confronted with one shared existential problem: how to keep themselves safe in the face of constant exposure to harmful microorganisms. Mounting evidence suggests that plants and animals have independently evolved similar receptors that sense pathogen molecules and set in motion appropriate innate immune responses. Now, in a study just published in the journal Cell Host & Microbe, senior author Takaki Maekawa together with co-first authors Lisa K. Mahdi, Menghang Huang, Xiaoxiao Zhang and colleagues have discovered that plants have evolved a family of proteins that bear a striking resemblance to proteins called mixed lineage kinase domain-like proteins (MLKLs), which trigger cell death in vertebrates as part of the immune response. In uncovering and characterizing an important new family of plant immune proteins, the authors’ study, which involved collaboration with fellow MPIPZ researchers Paul Schulze-Lefert, Jane Parker and Jijie Chai, provides intriguing new insights into how plants protect themselves from microbial invaders.
Regulated cell death often accompanies immunity against infection in plants, animals and fungi. One pervasive theory suggests that highly localised cell death responses serve to strictly limit the spread of infection. Although starting from independent origins this shared response seems to also involve highly similar machinery: many proteins involved in cell death in different kingdoms of life contain a so-called HeLo domain, a bundle structure made up of four helices, which causes resistance and cell death by disturbing the integrity of cellular membranes or forming ion channels.
Based on the similarities between animal and plant immune systems and on the key role played by HeLo domains in cell death, Maekawa hypothesised that plants might also contain other proteins with HeLo domains. Making use of bioinformatic and structural analysis, he and his team discovered a new family of HeLo domain-containing proteins that are widely shared among different plant species, indicating that they are important for plant physiology.
Maekawa termed the proteins plant MLKLs, and for further studies he focused on MLKLs expressed in the model plant Arabidopsis thaliana. He and his team isolated MLKL proteins from A. thaliana and determined that plant MLKLs possess the same overall protein architecture as their vertebrate counterparts and also assemble into tetramer, likely auto-inhibited, structures when they’re not active. Importantly, plant MLKLs also play a role in immunity, as plants in which genes encoding these proteins were mutated and thus non-functional were susceptible to pathogen infection.
Further investigation revealed additional similarities with vertebrate MLKLs: plant MLKLs are also trafficked to cellular membranes as part of their function, and activation of these proteins leads to cell death. Maekawa now aims to discover the molecular details underlying the function of plant MLKLs in immunity: “It will be exciting to uncover exactly how MLKLs are activated upon pathogen infection and how this activation is translated into effective plant protection.”
